Iwilfin (eflornithine) – Oral Maintenance Therapy for High‑Risk Neuroblastoma
What Is Iwilfin?
Iwilfin Eflornithine is a prescription oral medication whose active ingredient, eflornithine, has received FDA approval on December 13, 2023 for reducing the risk of relapse in adult and pediatric high‑risk neuroblastoma (HRNB) patients who have responded at least partially to prior multimodal treatment, including anti‑GD2 immunotherapy.
How It Works & Clinical Impact
Iwilfin is a targeted therapy—an irreversible inhibitor of the enzyme ornithine decarboxylase (ODC)—that disrupts polyamine synthesis and suppresses oncogenic drivers like MYCN and LIN28B, which play key roles in neuroblastoma progression.
Clinical evidence demonstrates a 52 % reduction in relapse risk and significant improvement in event‑free survival (EFS) and overall survival (OS) compared to similar patients without Iwilfin.
Usage & Dosage Guidelines
-
Administration: Twice daily (BID), with or without food.
-
Form: 192 mg oral tablets
-
Duration: Up to 2 years, or until disease progression or unacceptable toxicity.
-
Special dosing:
-
Dosage calculated by body surface area (BSA)
-
Tablets may be swallowed whole, chewed, or crushed and mixed with soft food or liquid—consume full mixture within 1 hour.
-
Baseline tests (CBC, liver function, hearing evaluation) are required pre‑treatment and periodically during therapy.
-
Safety & Side Effects
Warnings & Precautions:
-
Myelosuppression—monitor blood counts; may lead to neutropenia, anemia, thrombocytopenia.
-
Hepatotoxicity—liver enzymes may elevate; periodic liver monitoring needed.
-
Ototoxicity—hearing loss is known and sometimes serious; audiograms are mandatory.iwilfin.comDrugs.com
Common Side Effects:
Ear infection, diarrhea, sinus or respiratory infections, vomiting, fever, pink eye, upper respiratory issues, skin or urinary tract infections.
Reproductive Considerations:
-
Unknown safety in pregnancy; use effective contraception during treatment and for 1-week afterward.
-
Avoid breastfeeding during treatment and for 1-week after.
Accessibility & Specialty Pharmacy Support
-
Specialty Pharmacy Distribution: Iwilfin must be obtained via specialty pharmacies, not typical retail outlets.i
-
Patient Support via IWILFIN Cares™:
-
Assistance with insurance coverage evaluations
-
Financial support eligibility checks
-
Coordinated home delivery services
-
24/7 access to pharmacists for urgent questions
-
Refill reminders and educational resources
-
Regulatory Status & Availability
-
FDA: Approved in the U.S. on December 13, 2025, for HRNB therapy.
-
Europe (EMA): As of March 2024, no active application for EMA approval; availability in Europe remains limited/unlikely in the near term.
Storage Instructions
Store at room temperature (20–25 °C / 68–77 °F). Keep out of children’s reach.
FAQ
Q: What is Iwilfin used for?
A: Iwilfin is an oral maintenance medication approved to reduce relapse risk in high‑risk neuroblastoma patients who responded to prior treatment.
Q: How effective is Iwilfin?
A: It reduced relapse risk by around 52% and significantly improved survival rates in clinical studies.
Q: How do you take Iwilfin if you can’t swallow tablets?
A: Chew or crush and mix with soft food or liquid; consume the entire mixture within one hour.
Q: Who can dispense Iwilfin?
A: It’s dispensed through specialty pharmacies, supported by the IWILFIN Cares™ program that assists with insurance, delivery, financial aid, and pharmacist access.
Q: Is Iwilfin available in Europe?
A: As of early 2024, it’s not EMA-approved and thus not widely available in Europe yet.
Q: Can pregnant women use Iwilfin?
A: Safety during pregnancy is not established—effective contraception is required during and for one week after treatment.
Q: How should I store Iwilfin?
A: Store at room temperature (20–25 °C / 68–77 °F), away from children.

 Best stimulant drugs
Best stimulant drugs Medical Psychedelics
Medical Psychedelics Pharmaceutical weight loss drugs
Pharmaceutical weight loss drugs Steroids for sale
Steroids for sale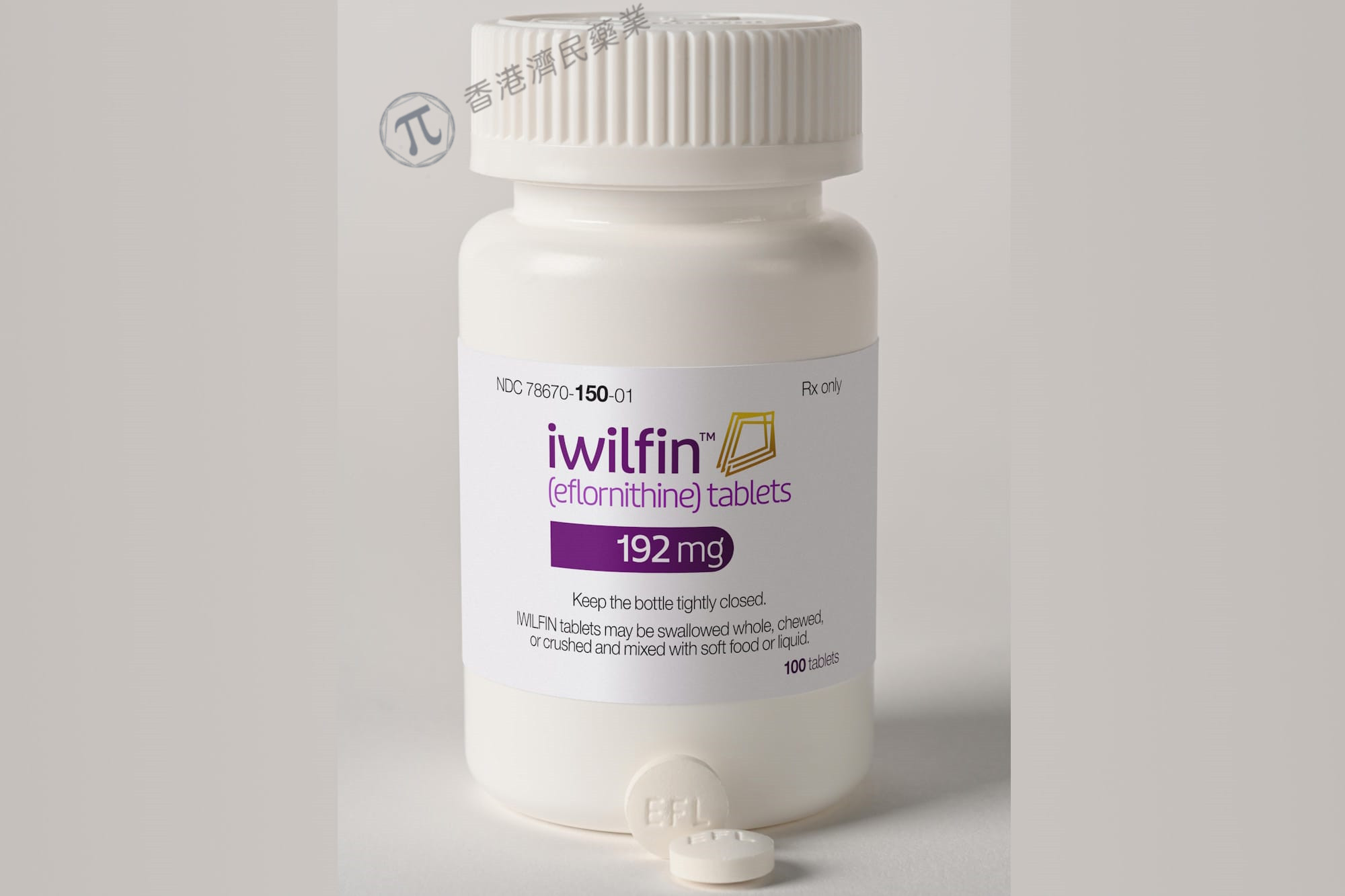











Reviews
There are no reviews yet.